B-hAREG MC38
|
Common name |
B-hAREG MC38 | Catalog number | 311648 |
| Aliases |
ARB, CRDGF, SDGF, AREG |
Disease | Colon carcinoma |
|
Organism |
Mouse |
Strain | C57BL/6 |
| Tissue types | Colon | Tissue | Colon |
Description
The mouse Areg gene was replaced by human AREG coding sequence in B-hAREG MC38 cells. Human AREG is highly expressed in the suspensions of B-hAREG MC38 cells.
Gene targeting strategy for B-hAREG MC38 cells. The exogenous promoter and chimeric AREG coding sequence containing human extracellular domain, mouse transmembrane domain and cytoplasmic domain were inserted to replace part of murine exon 2 and all of exon 3 and exon 4. The insertion disrupts the endogenous murine Areg gene, resulting in a non-functional transcript.
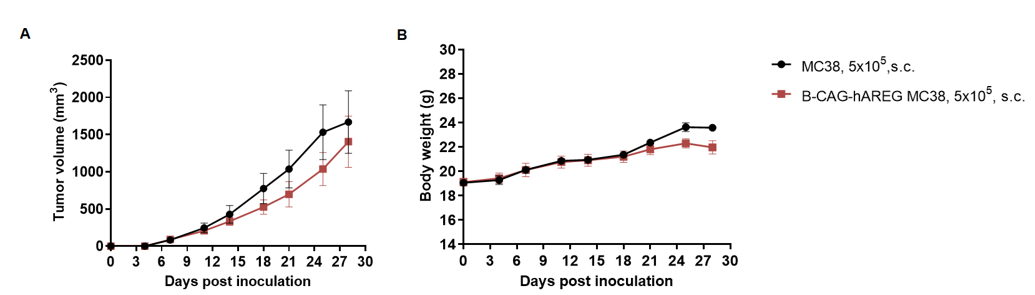
Subcutaneous homograft tumor growth of B-hAREG MC38 cells. B-hAREG MC38 cells (5x105) and wild-type MC38 cells (5x105) were subcutaneously implanted into C57BL/6N mice (female, 8-week-old, n=5). Tumor volume and body weight were measured twice a week. (A) Average tumor volume ± SEM. (B) Body weight (Mean± SEM). Volume was expressed in mm3 using the formula: V=0.5 X long diameter X short diameter2. As shown in panel A, B-hAREG MC38 cells were able to establish tumors in vivo and can be used for efficacy studies.
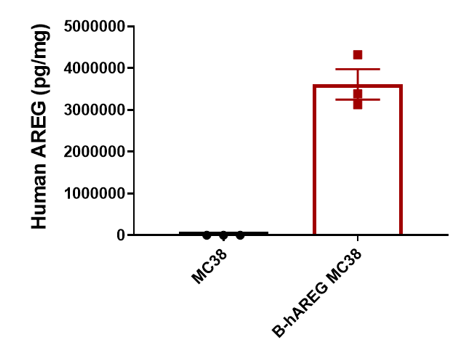
B-hAREG MC38 cells were subcutaneously transplanted into C57BL/6N mice (n=5), and on 33 days post inoculation, tumor cells were harvested and assessed for human AREG expression by ELISA. As shown, human AREG was highly expressed in the suspensions of tumor cells. Therefore, B-hAREG MC38 cells can be used for in vivo efficacy studies of novel AREG therapeutics.







