YH008 is a tetravalent PD1×CD40 Fc-silenced IgG1 bispecific antibody (BsAb) that agonizes CD40 by cross-linking through PD1. This product utilizes Biocytogen’s rapid and efficient antibody development platform, complemented by an in vivo pharmacodynamic evaluation process, demonstrates both efficay and safety profiles.
We have FDA IND cleared in Decemeber 2022 and NMPA IND cleared in March 2023. Our partner is conducting a Phase I clinical study in china, which has enrolled patients with late stage solid tumors for a dose-escalation study.

YH008 Format
Pre-clinical study results:
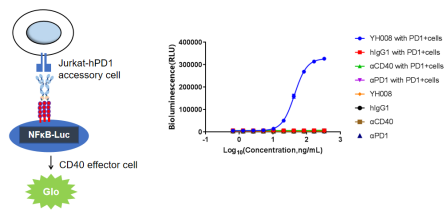
1.YH008 requires PD-1 expression to activate human CD40 signaling.
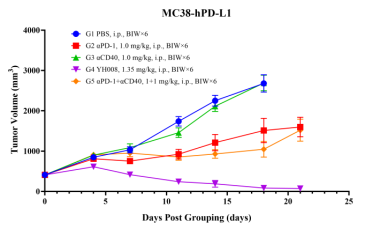
2. YH008 shows superior potency than parental mAbs in MC38 syngeneic models.
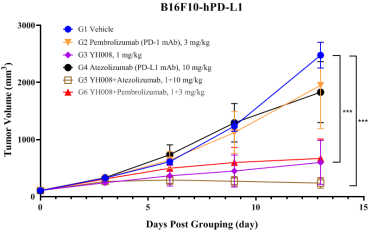
3.YH008 demonstrates robust in vivo anti-tumor efficacy in a PD-1/-L1 antibodies resistance tumor model.
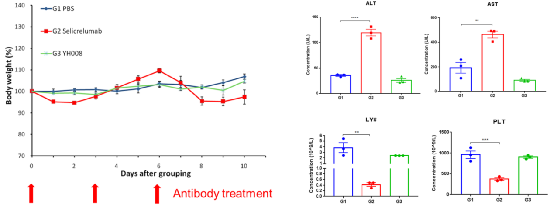
4.YH008 shows better safety compared to Selicrelumab analog
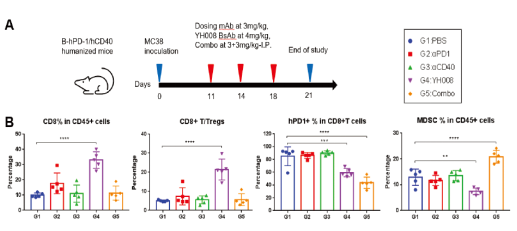
5.In the tumor microenvironment, YH008 increases the frequency of CD8+ T cells and reduces PD-1+ T cells and myeloid-derived suppressor cells.
Targets introduction
Programmed cell death protein 1 (PD-1) is a major inhibitor of T cell responses expressed on activated T cells. It is also expressed on natural killer cells, B cells, regulatory T cells, T follicular helper cells, and myeloid cells.
CD40 (TNFRSF5), belongs to a member of the tumor necrosis factor receptor (TNFR) super family, mainly expressed by DCs, myeloid cells and B cells. CD40 is a key stimulatory receptor that plays a critical role in antigen presentation (APC) and T cell activation. The binding to its ligand, CD40L (also called CD154), could activate NF-κB signaling and ‘licensing’ DCs to prime effective cytotoxic T-cell anti-tumor responses.
News
Poster Download








