
Biocytogen’s antibody BD and licensing (BDL) team will be delivering an oral presentation on Monday, June 5th at the 2023 BIO International Convention in Boston, taking place from June 5-8. The presentation will highlight Biocytogen’s clinical and preclinical pipeline assets, 6 fully human antibody discovery platforms, as well as the progress of Project Integrum, an initiative to develop therapeutic antibodies for more than 1000+ targets. Biocytogen’s BDL team will also host one-on-one partnering meetings at the event.
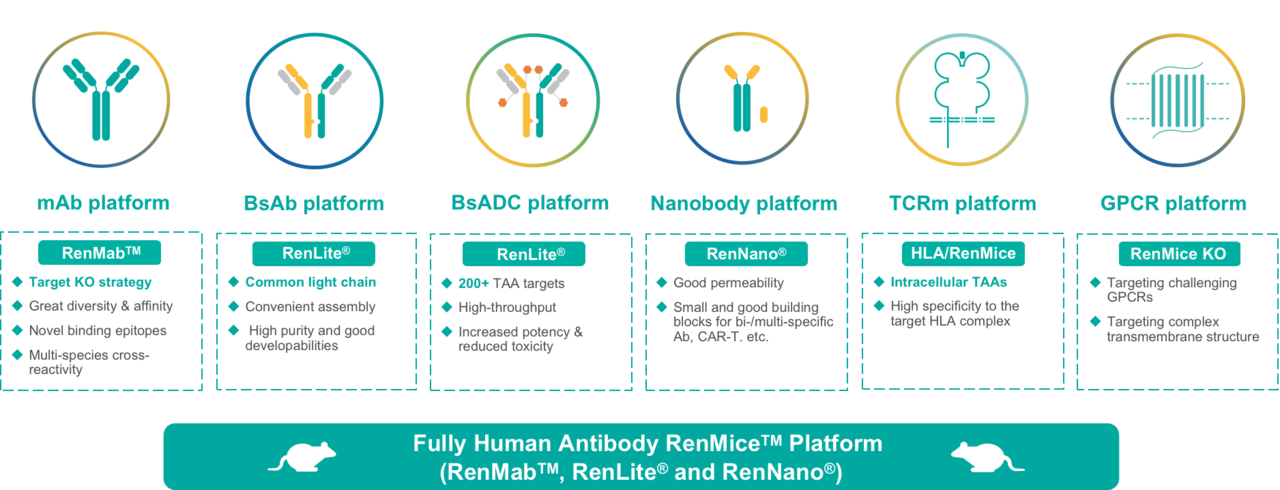
Using Biocytogen’s proprietary fully human antibody RenMiceTM, Biocytogen has built 6 antibody discovery platforms:
- monoclonal antibody (mAb) platform: RenMabTM-based monoclonal antibody discovery with high affinity and great diversity;
- bispecific antibody (BsAb) platform: RenLite®-based common light chain bispecific antibody discovery with high pairing success rate and good druggability;
- bispecific ADC (BsADC) platform: high-throughput RenLite®-based bispecific ADC discovery for 200+ TAA targets with good tumor specificity, anti-tumor activity, and CMC efficiency;
- nanobody platform: RenNano®-based single-domain antibody (nanobody) discovery with great blood-brain-barrier permeability and tumor infiltrating ability, suitable for the assembly of bispecific, multi-specific antibodies, CAR-T therapies, etc.
- TCR-mimic antibody platform: HLA-transgenic RenMice-based TCR-mimic (TCRm) antibody divscovery for intracellular targets with superior affinity and specificity;
- GPCR platform: target knockout RenMice-based antibody discovery for multi-species cross-recognition of GPCR and other challenging targets;
With an established in vivo efficacy screening platform and streamlined antibody development capability, Biocytogen launched Project Integrum to develop therapeutic antibodies for more than 1000+ targets. Since the initiation of Project Integrum, the company has generated 6 clinical stage assets:
- YH003: CD40 mAb, which is currently in phase II MCRTs;
- YH001: CTLA-4 mAb in an ongoing phase I clinical trial in partnership with TRACON for the treatment of sarcoma;
- YH008: PD-1 × CD40 BsAb, which has received FDA and NMPA IND clearance, has been licensed in Greater China by Chipscreen NewWay Biosciences;
- YH002: OX40 mAb, which is in phase I clinical trials in Australia and China;
- YH004: 4-1BB mAb, which is in phase I clinical trials in Australia and China;
- YH005: Claudin 18.2 mAb which has been out-licensed to RemeGen to develop Claudin 18.2 ADC, known as RC118.
and numerous preclinical stage assets:
- 10+ fully human bispecific antibodies, including YH006 (CTLA-4 x OX40 BsAb);
- 20+ bispecific antibody-drug conjugates, including YH012 (HER2 x TROP2 BsADC), YH013 (EGFR x MET BsADC) and BSA01 (EGFR x MUC1 BsADC);
- 10+ TCR-mimic antibodies targeting intracellular TAAs, including WT-1, KRAS, P53, AFP, GP100 and NY-ESO-1;
- 80+ monoclonal antibodies targeting B7H3, TNFR2, CCR8, AMHR2 and other novel TAAs, co-stimulatory/co-inhibitory molecules, tumor microenvironment molecules, GPCRs, and other popular targets;
- 600+ antibody hits for oncology, inflammatory/autoimmune disease targets, infectious disease targets, and neurodegenerative disease targets, including B7H4, DLL3, MUC1, GPRC5D, PSMA, LRRC15, GUCY2C, LILRB2, NECTIN-4, ROR1, TIGIT and others.
Biocytogen’s antibody assets and RenMice platforms have been recognized by many pharmaceutical and biotech companies. As of December 31, 2022, Biocytogen has reached 34 preclinical antibody co-development or licensing agreements with 21 companies, including Merck KGaA, ADC Therapeutics, Hansoh Pharma, Chipscreen NewWay, LiberoThera, RemeGen, China Resources Biopharm and Nanjing Chia-Tai Tianqing Pharmaceutical Company (NJCTTQ). Additionally, the company has licensed RenMice (RenMab/RenLite) to 17 companies, including Merck KGaA, Janssen, BeiGene, Xencor and Innovent; 40 projects have initiated.
The team is looking forward to discussing licensing and co-development opportunities with you, either for antibody assets or fully human antibody discovery platforms. To schedule 1-on-1 partnering meetings, please visit https://www.bio.org/events/bio-international-convention/one-one-partneringtm. For questions, or more information, please contact BD-Licensing@biocytogen.com.








