| Strain Name |
C57BL/6-Tfrctm1(TFRC)Bcgen/Bcgen
|
Common Name | B-hTFR1 mice |
| Background | C57BL/6 | Catalog number |
110861 |
|
Related Genes |
T9; TR; TFR; p90; CD71; TFR1; TRFR; IMD46 |
||
|
NCBI Gene ID |
22042 | ||
mRNA expression analysis

Strain specific analysis of TFR1 gene expression in wild type (WT) mice and B-hTFR1 mice by RT-PCR. Mouse Tfrc mRNA was detectable only in splenocytes of WT mice (+/+). Human TFRC mRNA was detectable only in homozygous B-hTFR1 mice (H/H) but not in WT mice (+/+).
Protein expression analysis in erythroid cells

Strain specific TFR1 expression analysis in homozygous B-hTFR1 mice by flow cytometry. Bone marrow was collected from wild type (WT) mice (+/+) and homozygous B-hTFR1 mice (H/H), and analyzed by flow cytometry with species-specific anti-TFR1 antibody. Mouse TFR1 was detectable in WT mice (+/+). Human TFR1 was exclusively detectable in homozygous B-hTFR1 mice (H/H) but not in WT mice (+/+).
Protein expression analysis in brain
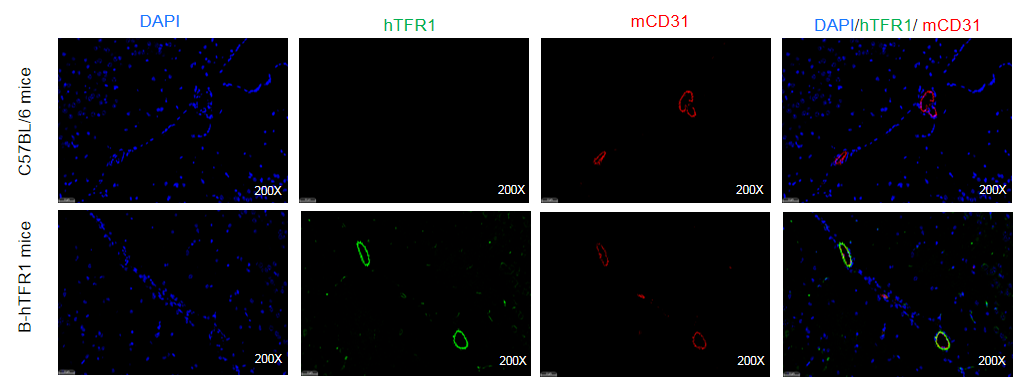
Strain specific TFR1 expression analysis in homozygous B-hTFR1 mice by Immunofluorescence staining. Brain was collected from wild-type C57BL/6 mice and homozygous B-hTFR1 mice (female,8-week-old) and processed into paraffin sections. The paraffin sections were stained using an species-specific anti-human TFR1 antibody (green). Brain tissues were co-stained with an anti-mouse CD31 antibody (red) to visualize microvascular endothelial cells. The results indicated that human TFR1 was detectable on brain microvascular endothelium of homozygous B-hTFR1 mice, but not in wild-type C57BL/6 mice.
Protein expression analysis in bone marrow
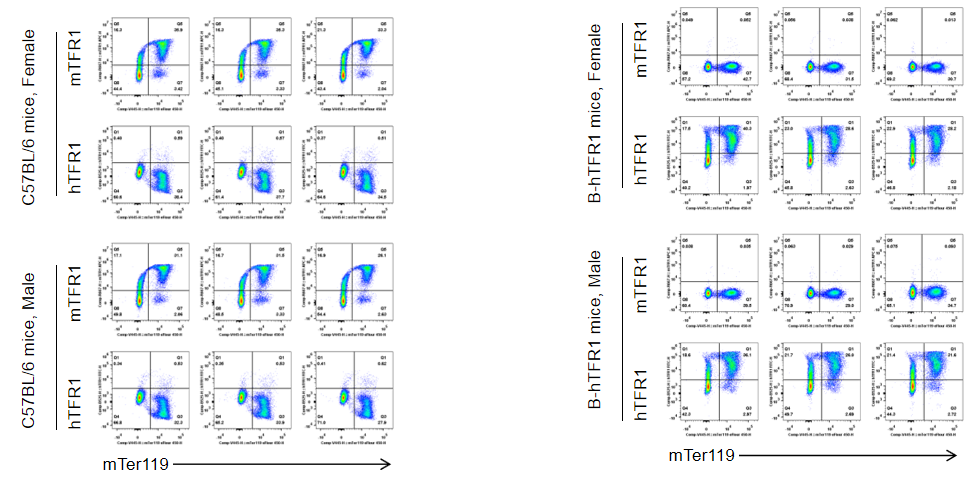
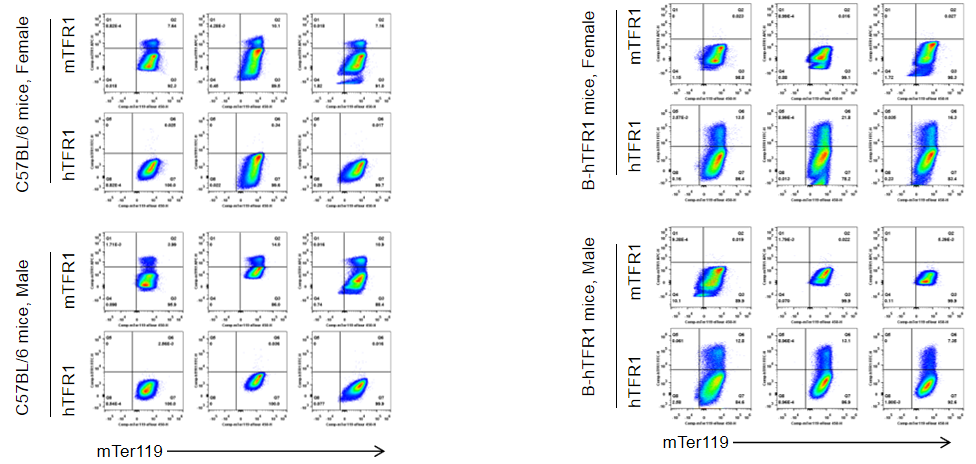
Strain specific TFR1 expression analysis in wild-type C57BL/6 mice and homozygous humanized B-hTFR1 mice by flow cytometry. Blood cells were collected from wild-type C57BL/6 mice and homozygous B-hTFR1 mice (male and female, 9-week-old, n=3). Protein expression was analyzed with anti-TFR1 antibody by flow cytometry. Mouse TFR1 was only detectable in wild-type C57BL/6 mice. Human TFR1 was exclusively detectable in homozygous B-hTFR1 mice, but not in wild-type C57BL/6 mice. The results showed that the expression level of human TFR1 in B-hTFR1 mice is gender independent, similar to the expression level of mouse TFR1 in wild-type C57BL/6 mice .
Protein expression analysis of TFR1 in brain endothelial cells

Strain specific TFR1 expression analysis in wild-type C57BL/6JNifdc and homozygous B-hTFR1 mice by flow cytometry. Brain cells were collected from wild-type C57BL/6JNifdc (+/+) and homozygous B-hTFR1 mice (H/H), and analyzed by flow cytometry with anti-mouse TFR1 antibody (Biolegend, 113808) and anti-human TFR1 antibody (Biolegend, 334108). mTFR1 was only detectable in brain endothelial cells in wild-type mice, and hTFR1 was exclusively detectable in homozygous B-hTFR1 mice but not in wild-type mice.
Protein expression analysis in brain

Human TFR1 was detectable on brain microvascular endothelium of homozygous B-hTFR1 mice, but not in wild-type C57BL/6 mice.
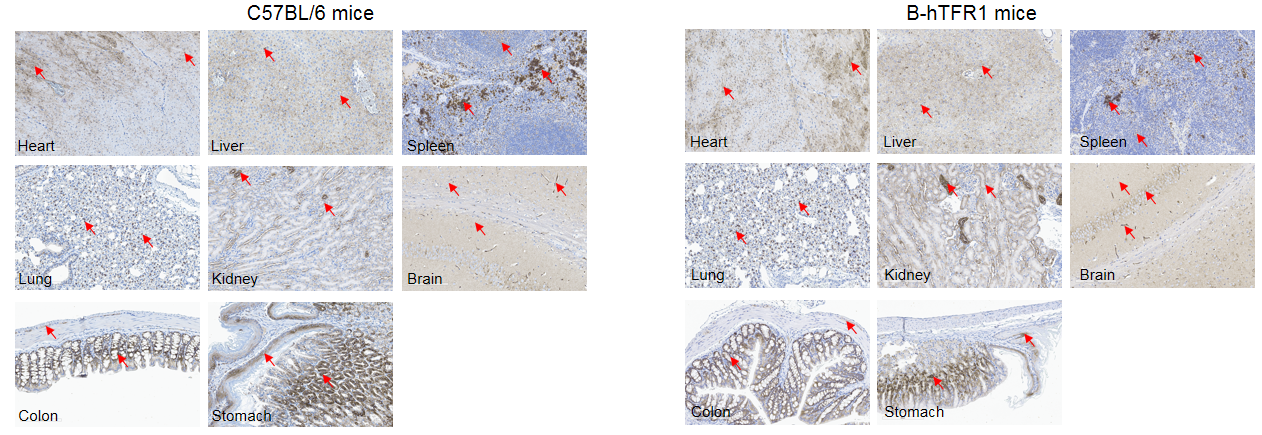
Immunohistochemical (IHC) analysis of TFR1 protein expression in wild-type C57BL/6 mice and homozygous humanized B-hTFR1 mice. Mouse tissues were collected from wild-type C57BL/6 mice and homozygous B-hTFR1 mice (female, 8-week-old, n=3). Protein expression was analyzed with anti-TFR1 antibody by IHC. Human TFR1 was detected in the heart, liver, spleen, lung, kidney, brain, colon and stomach of B-hTFR1 mice, which is similar to the expression pattern of mouse TFR1 in wild-type C57BL/6 mice. Red arrow: positive cells expressing TFR1.
Protein expression analysis of B-hTFR1 mice in multiple tissues

Western blot analysis of TFR1 protein expression in wild-type C57BL/6JNidc mice and homozygous B-hTFR1 mice by WB. Various tissues were collected from wild-type C57BL/6JNifdc mice (+/+) and homozygous B-hTFR1 mice (H/H), and then analyzed by western blot with anti-TFR1 antibody (abcam, ab214039). 40 μg total proteins were loaded for western blotting analysis. GAPDH were detected as internal control. TFR1 was detectable in heart, liver, spleen, lung, kidney, stomach, colon, muscle and brain from both C57BL/6JNifdc and homozygous B-hTFR1 mice, as the antibody was cross-reactive between human and mouse. M, marker.
Protein expression analysis of TFR1 in eyes

TFR1 expression analysis in eyeball. The expression of human TFR1 was observed in corneal epithelial cells, endothelial cells, and lens epithelial cells, while it was highly expressed in the retina in B-hTFR1/hCD98HC mice, but not in wild-type C57BL/6 mice.
mRNA expression analysis of TFR1 in different ages of mice

TFR1 mRNA expression analysis in homozygous B-hTFR1 mice. Brain (A), skeletal muscle (B), eyeball (C) and heart (D) RNA were isolated from wild-type C57BL/6 mice (+/+) (female, 7-week-old, n=3; male, 7-week-old and 24-week-old, n=3) and homozygous B-hTFR1 mice (H/H) (female, 7-week-old, 11-week-old, 15-week-old, n=3; male, 7-week-old, 11-week-old, 15-week-old, 24-week-old, n=3) ), then cDNA libraries were synthesized by reverse transcription, followed by PCR with TFR1 primers. TFR1 expression level in female homozygous B-hTFR1 mice is similar to those in wild-type C57BL/6 mice and remains consistent across different ages of mice (up). TFR1 expression level in male homozygous B-hTFR1 mice is similar to those in brain, heart and eyeball in wild-type C57BL/6 mice (down). Values are expressed as mean ± SEM.
Analysis of leukocytes cell subpopulations in B-hTFR1 mice

Humanization of TFR1 does not change the overall frequency or distribution of immune cell types in spleen, blood and lymph nodes. Values are expressed as mean ± SD.


Liver complement component 3 levels in wild-type C57BL/6 and homozygous humanized B-hTFR1 mice. Liver were collected from C57BL/6 and B-hTFR1 mice (n=3, 6 week old, female), then cDNA libraries were synthesized by reverse transcription, followed by qPCR with mouse complement component 3 primers. The mRNA expression of mouse complement component 3 in homozygous B-hTFR1 mice was similar to those in the wild-type C57BL/6 mice. Values are expressed as mean ± SEM. Significance was determined by unpaired t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
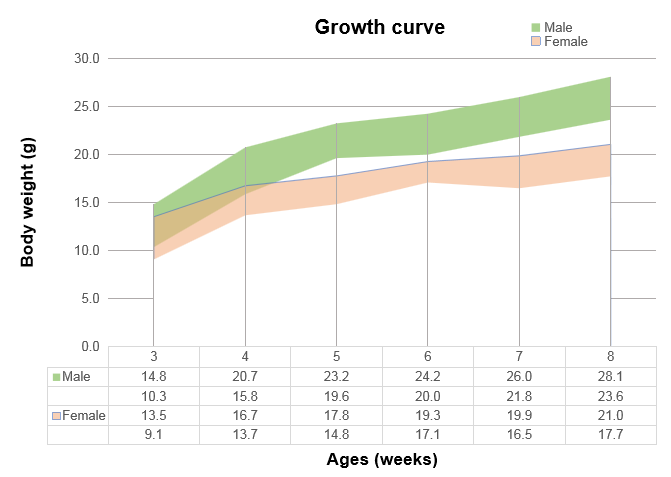

Humanization of TFR1 does not alter hematological parameters. Values are expressed as mean ± SD.
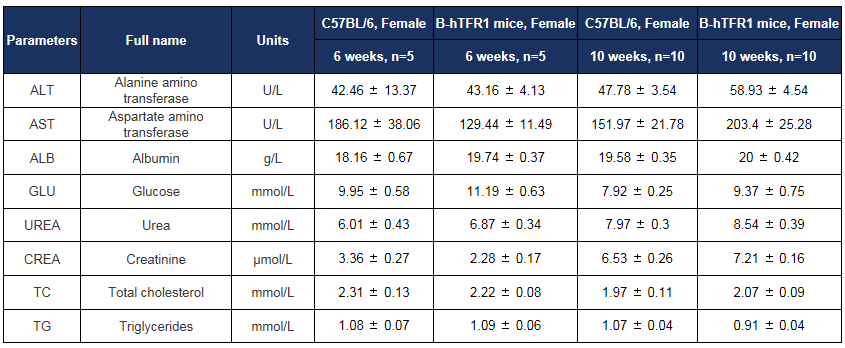
Biochemical test of B-hTFR1 mice. Values are expressed as mean ± SD.
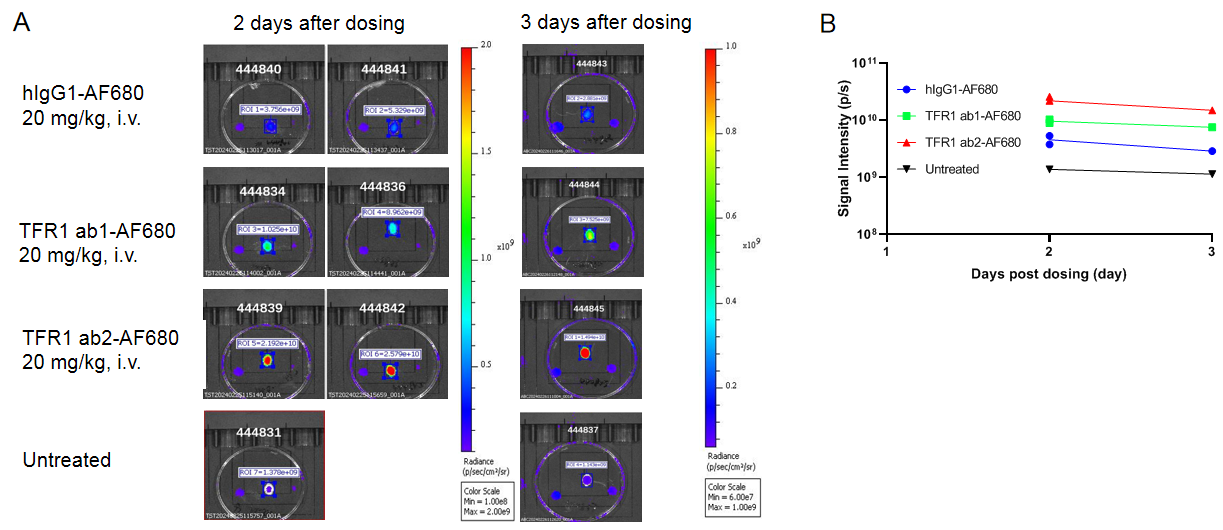
In vivo PK evaluation of anti-human TFR1 BsAbs

In vivo pharmacokinetic (PK) evaluation of anti-human TFR1 bispecific antibodies (BsAbs). B-hTFR1 mice were injected with control IgG (10 mpk) and anti-human TFR1 BsAbs (10.9 mpk) provided by a client via tail vein. Brain and serum were taken for in vivo PK evaluation. Brain concentrations(A), serum concentrations (B), and brain-to-serum ratio (C) of anti-human TFR1 BsAbs were quantified. As shown in panel, anti-human TFR1 BsAbs exhibited higher serum clearance and enhanced brain exposure after dose. The results confirmed that brain of B-hTFR1 mice enables uptake of an intravenously administered anti-human TFR1 BsAbs and B-hTFR1 mice provide a powerful preclinical model for in vivo evaluation of effective delivery of protein therapeutics to the central nervous system (CNS). Graphs represent mean ± SEM.
Note: This experiment was performed by the client using B-hTFR1 mice. All the other materials were provided by the client.








