RA(Rheumatoid Arthritis) /CIA(Collagen—Induced Arthritis) Mouse Model
Rheumatoid Arthritis (RA) is one of the most common autoimmune diseases in human, and its clinical symptoms mainly include generalized joint swelling, pain and motion disorder. In severe cases lead to disability. The pathology mainly showed hyperplastic synovitis, cartilage injury and bone structure destruction. In order to study rheumatoid arthritis disease better, researchers have developed many animal models. During those models, collagen-induced arthritis (CIA) is an experimental autoimmune disease that can be elicited in susceptible strains of rodents (rat and mouse) primates by immunization with type II collagen(CII), the major constituent protein of articular cartilage. Following immunization, these animals develop autoimmune polyarthritis that shares several clinical and histological features with rheumatoid arthritis.
Biocytogen has established stable CIA disease models in different strains of mice (C57BL/6, DBA), which can be used for pharmacodynamic evaluation of rheumatoid arthritis related drugs.
Establishment of CIA Mouse Model
Experimental Animals:C57BL/6 or DBA1,10-13 weeks old, male
Modeling reagent:CII emulsion
Modeling method:Sensitization—0 days
Challenge—21 days
Clinical Score
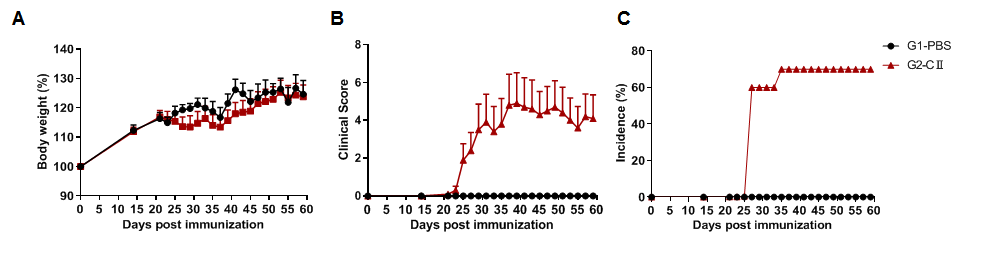
The arthritis model was induced in C57BL/6 mice using collagen (CII). (A) mouse body weight change; (B) clinical score; (C) incidence of morbidity, defined by (add symptoms). The results showed that the clinical score of G2 group mice was significantly increased, suggesting that the arthritis model was successfully established.
Pathological analysis
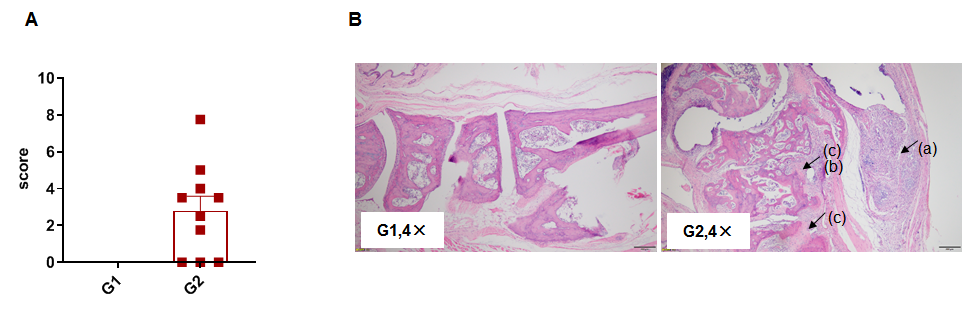
Pathological analysis after the establishment of arthritis in C57BL/6 mice. (A) Pathological score; (B) H&E staining of pathological sections. In the model group, subcutaneous mixed inflammatory cell infiltration (a), periarticular stenosis (b), articular cartilage and bone tissue destruction (c) and other arthritic lesions were observed in all or part of the limb joints, suggesting that the arthritis model was successfully established.
Efficacy Validation on CIA (wild-type C57BL/6) Mouse Models for Methotrexate
Clinical Score
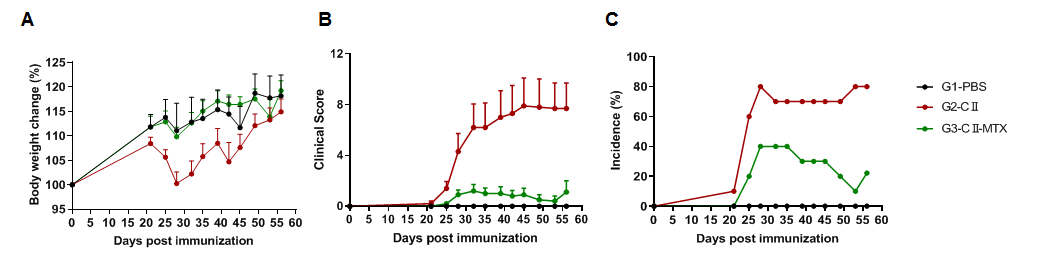
Pharmacodynamic effects of methotrexate in a mouse arthritis model. (A) mouse body weight change; (B) clinical score; (3) mouse morbidity. The results showed that in the G2 model group (G2), the clinical scores of mice were significantly increased, indicating that the CIA model was successfully established, and the body weight fluctuation was more obvious than that in the control group (G1). After administration of methotrexate (MTX), the mean clinical score of the treatment group (G3) was significantly lower than that of the G2 group, indicating that the disease was effectively controlled. In addition, in terms of incidence, the maximum incidence in the treatment group was 40%, which was also significantly lower than the incidence in the modeling group of nearly 80%, suggesting that the small molecule drug methotrexate alleviates disease symptoms.
Pathological analysis
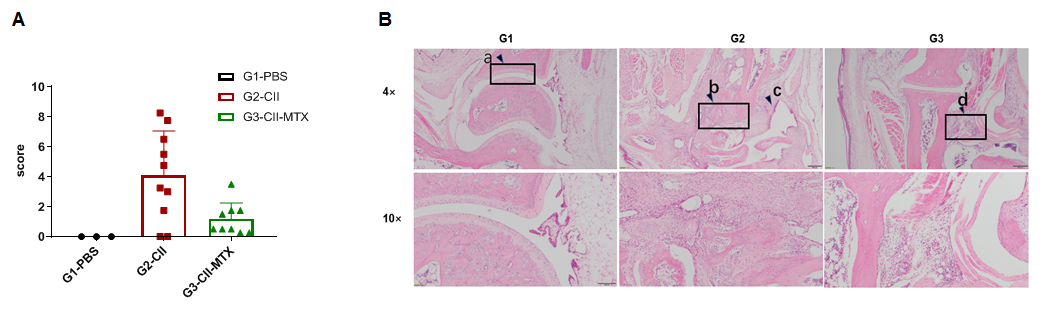
Pathological analysis of the pharmacodynamic effects of methotrexate in a mouse arthritis model. (A) Pathological score; (B) H&E staining of pathological sections. Pathological results showed that the animals in the control group (G1) had significant abnormal changes, the surface of ankle cartilage was smooth, and the articular cavity was obvious (a). In the model group (G2), subcutaneous mixed inflammatory cell infiltration, synovitis and/or pannus formation (c), articular cartilage destruction, disappearance of joint cavity and partial bone tissue fusion (b) were observed in the tissues around the ankle joints. Although there was edema and inflammatory cell infiltration (d) in the subcutaneous tissue around some ankles in the treatment G3 group, the mean pathological score was significantly lower than that in the G2 group, and methotrexate exhibited a therapeutic effect on arthritic lesions in animals.
B-hIL6/hIL6R mice
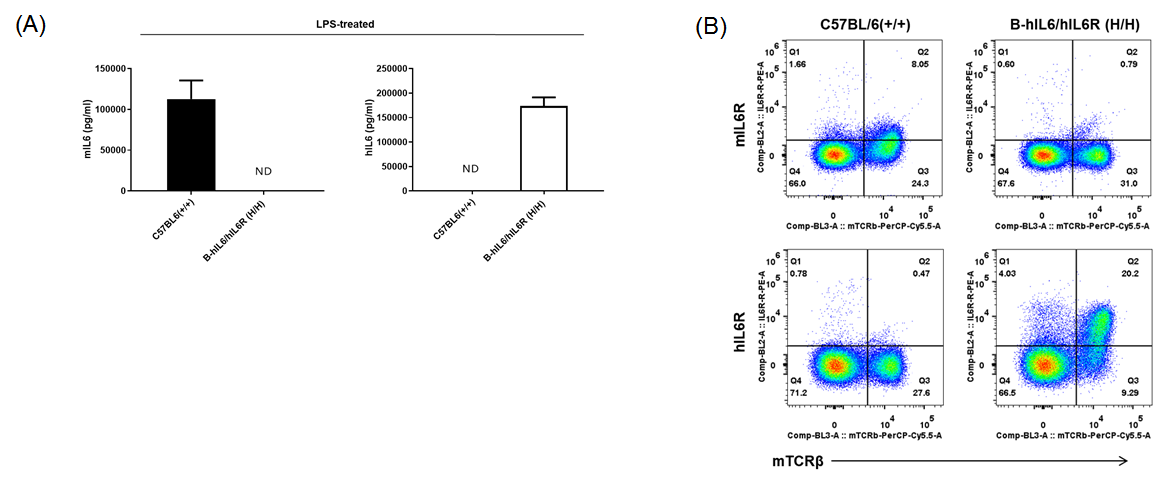
Strain specific IL6 and IL6R expression analysis in homozygous B-hIL6/hIL6R mice. (A) Serum was collected from WT (+/+) and homozygous B-hIL6/hIL6R mice (H/H) stimulated with LPS in vivo, and analyzed by ELISA with species-specific IL6 ELISA kit. Mouse Il6 was detectable in WT mice. Human IL6 was exclusively detectable in homozygous B-hIL6/hIL6R mice but not in WT mice. (B) Splenocytes were collected from WT (+/+) and homozygous B-hIL6/hIL6R mice (H/H), and analyzed by flow cytometry with species-specific anti-IL6R antibody. Mouse IL6R was detectable in WT mice. Human IL6R was exclusively detectable in homozygous B-hIL6/hIL6R mice but not in WT mice.
Efficacy Validation on CIA (B-hIL6/hIL6R mice) Mouse Models for Sirukumab (in house)
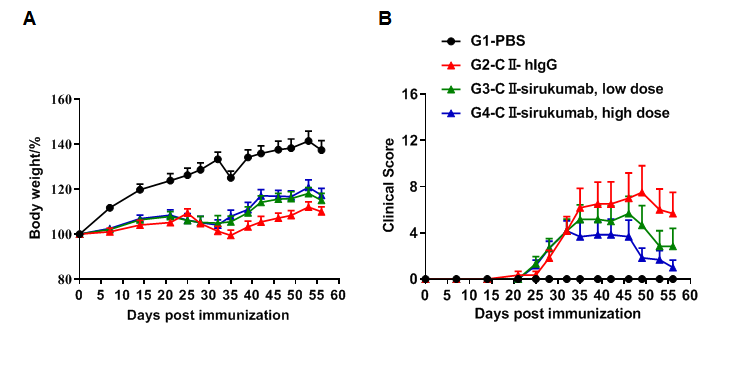
Pharmacodynamic effects of the anti-human IL-6 antibody sirukumab (in house) in an arthritis mouse model. (A) mouse body weight change; (B) clinical score. The results showed that after the model was successfully established, mice in the treatment group (G3, G4) treated with sirukumab antibody showed improved clinical scores in a dose-dependent manner.
Pathological analysis
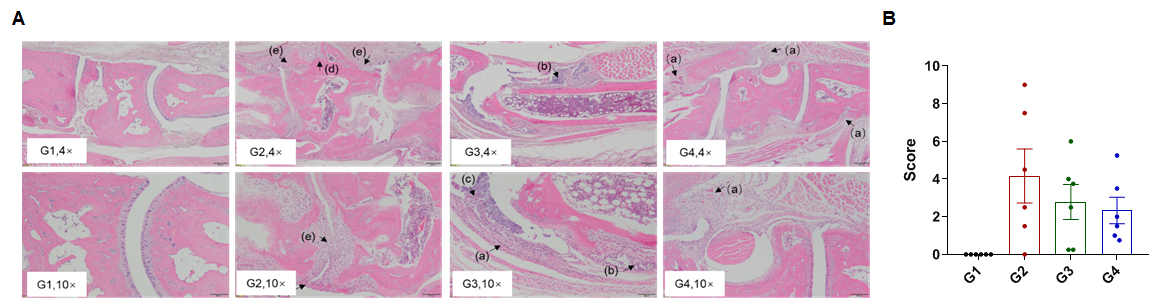
Efficacy of anti-human IL6 antibody in B-hIL6/hIL6R mice with collagen induced arthritis (CIA) model. Histopathological examination was performed on the joints of the extremities at endpoint. (A) Hematoxylin and eosin (H&E) staining. (B) Score of arthritis histology. G1 showed no significant abnormal changes. G2 showed bone structure damage(d), articular cavity or periarticular space disappeared (e), and pannus were observed (a), suggesting that the CIA model was successfully established. Compared with the G2 group, the low-dose group (G3) showed slight inflammatory cell infiltration (b) and pannus (a) and synovial hyperplasia (c). However, in the high dose group (G4), there was only partial pannus, the arthrosis disappeared and the articular cavity was obvious.
Product list
|
Product name |
Product number |
|
B-hIL6/hIL6R mice |
120557 |
References
1. Tateiwa, D., Yoshikawa, H. & Kaito, T. Cartilage and Bone Destruction in Arthritis: Pathogenesis and Treatment Strategy: A Literature Review. Cells 8, doi:10.3390/cells8080818 (2019).
2. McInnes IB, et al. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7, 429-42 (2007).







