Experimental Animals:C57BL/6, 10-13 weeks old, female
Modeling reagent:MOG emulsion and PTX
Modeling method:Immunized with MOG emulsion and injected pertussis toxin intraperitoneally
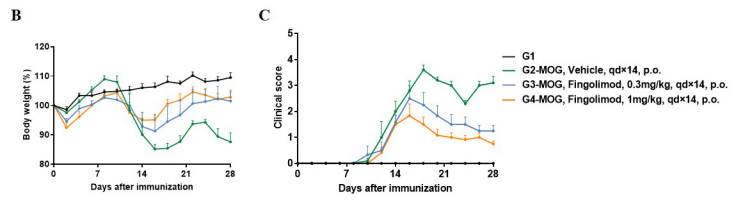
In vivo small molecule efficacy in C57BL/6 EAE model induced by MOG35-55 emulsion and PTX.
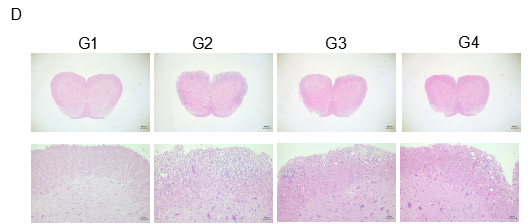
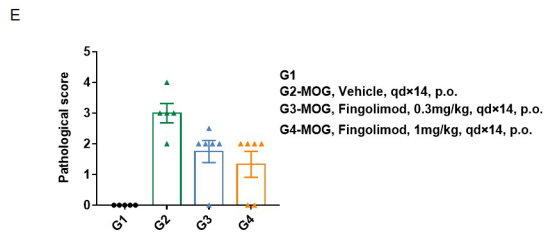
Local inflammatory responses in the central nervous system (CNS) of EAE mice.
Product list
|
Product name |
Product number |
|
B-hIL17A mice |
110053 |
|
B-hTNFA mice |
110002 |
References
1. Gaffen, S.L., Jain, R., Garg, A.V. & Cua, D.J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 14, 585-600 (2014).
2. Iwakura, Y. & Ishigame, H. The IL-23/IL-17 axis in inflammation. J Clin Invest 116, 1218-1222 (2006).
3. Kuwabara, T., Ishikawa, F., Kondo, M. & Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediators Inflamm 2017, 3908061 (2017).







